If the end product is liquid and gaseous hydrocarbons, the CH4 content has to be lown and both the Hydrogen and CO content have to be high. On the one hand the H2 has to be minimum of two times higher that the CO content, otherwise the liquefaction proces is not made effectively. In the early stages of the development and application of normal gasification systems the efficiency was low and the process just happened, because since the process management system only monitored the process, but could not influence it or keep it at the optimal point of creation from the point of view of the final products.
The liquefaction plant is one of the „end product units” that can be an integral part of the TCG-UC Plant. These kind of units are designed and manufactured, implemented and maintenanced by one of our subcontractors participating in the project.
This unit can produce any liquid hydrocarbon which are „green” or „blue” category, because both the manufacturing plant and the process itself is “environmental friendly” and in addition to all this feed material is dangerous to the environment.
„The concept of Fischer–Tropsch Technology originated at the beginning of the 20th Century when French Scientists Sabatier and Sanders prescribed a first of its kind process to produce methane from syngas (CO+H2) using Cobalt, Iron and Nickel catalyst. In 1923 renowned Scientist professor Franz Fischer, director of “Kaiser-Wilhelm Institute of Coal research” in Mulheim an der Ruhr along with Head of Department, Dr. Hans Tropsch discovered a synthesis to produce longer chain hydrocarbons which can be refined to yield gasoline, kerosene or diesel known as Fischer–Tropsch (F–T) Method. The Fischer–Tropsch technique produces longer-chain molecules of hydrocarbon from polymerization of syngas (CO+H2). By-products are carbon dioxide emission and production of steam or water. Figure below illustrates the overall schematic of Fischer–Tropsch technology. The syncrude composition from Fischer–Tropsch synthesis is basically govern:ed by catalyst types, the operating regime, other supplementary factors like catalyst promoters, reactor designs and Syngas composition (various ratios of H2:CO).”
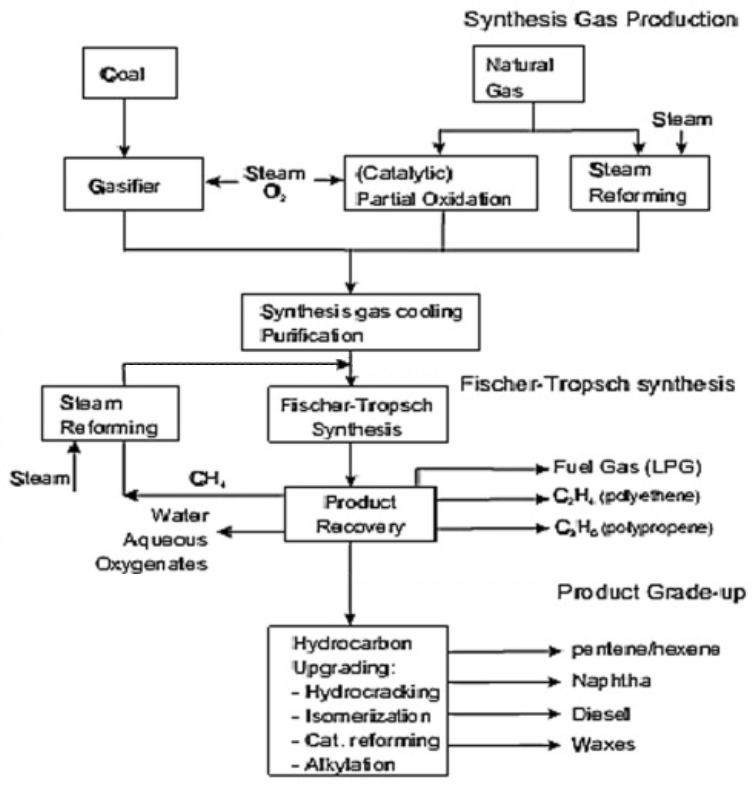
„The essential step, known as the Fischer-Tropsch (FT) reaction, can be written as
CO + (2n+1)H2 → CnH(2n+2) + nH2O
where CnH(2n+2) represents a range of hydrocarbons, ranging from low-molecular-weight gases (n=1, methane), by way of gasoline (n=5-12), diesel fuel (n=13-17), and as far as solid waxes (n>17). The FT reaction is usually a catalytic reaction at high temperatures (180-350oC) and high pressure for realistic rates to be achieved, and the typical catalysts used are based on iron or cobalt. The hydrocarbons produced by FT process can be refined and used in place of more conventional liquid fuels derived from crude oil. Synthetic fuel can be produced by a variety of gasification methods, with gas-to-liquid (GTL), coal-to-liquid (CTL) and biomass-to-liquid (BTL) being the most widespread.”