The Liquefaction in this meaning is a process when the synthesis gas - consisting of four gases - is used for manufacturing synthetique liquid hydrocarbon. These liquid “end product” can be any petrol, diesel, Jet A1, or other liquified hydrocarbon depending upon the length of the CH2 chain elements.
In this kind of liquefaction process the most important thing is the quantity of both the CO and the H2 molecules, but if the end product is the electricity, the H2 content has to be very low, and the CH4 content has to be raised.
This above listed two contrary expectations are the explanation that there is no such kind of gasification technology except the TCG. The known gasification technologies can not determin, or influence to a considerable extent the ratios of the gas components in the syngas, consequently there can not be used the same gasification plant/equipment for both to manufacture electric energy and liquid hydrocarbons.
This was the situation before the TCG. The TCG can be adjusted - and the ratios of the gas components of the syngas produced can be determined - depending upon the optimal ratio request from the point of view of the end procuct.
Reaction mechanism
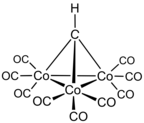
Methylidyne-tricobalt-nanocarbonyl is a molecule that illustrates the kind of reduced carbon species speculated to occur in the Fischer–Tropsch process.
The Fischer–Tropsch process involves a series of chemical reactions that produce a variety of hydrocarbons, ideally having the formula (CnH2n+2). The more useful reactions produce alkalines as follows:
(2n + 1) H2 + n CO → CnH2n+2 + n H2O
where n is typically 10–20. The formation of methane (n = 1) is unwanted. Most of the alkanes produced tend to be straight-chain, suitable as diesel fuel. In addition to alkane formation, competing reactions give small amounts of alkenes, as well as alcohols and other oxygenated hydrocarbons.
The reaction is a highly exothermic reaction due to a standard reaction enthalpy (ΔH) of −165 kJ/mol CO combined.
Fischer–Tropsch intermediates and elemental reactions
Converting a mixture of H2 and CO into aliphatic products is a multi-step reaction with several intermediate compounds. The growth of the hydrocarbon chain may be visualized as involving a repeated sequence in which hydrogen atoms are added to carbon and oxygen, the C–O bond is split and a new C–C bond is formed. For one –CH2– group produced by CO + 2 H2 → (CH2) + H2O, several reactions are necessary:
- Associative adsorption of CO
- Splitting of the C–O bond
- Dissociative absorption of 2 H2
- Transfer of 2 H to the oxygen to yield H2O
- Desorption of H2O
- Transfer of 2 H to the carbon to yield CH2
The conversion of CO to alkanes involves hydrogeneration of CO, the hydrogenolysis (cleavage with H2) of C–O bonds, and the formation of C–C bonds. Such reactions are assumed to proceed via initial formation of surface-bound metal carbonyls. The CO ligand is speculated to undergo dissociation, possibly into oxide and carbide ligands. Other potential intermediates are various C1 fragments including formyl (CHO), hydroxycarbene (HCOH), hydroxymethyl (CH2OH), methyl, (CH3), methylene (CH2), methylidyne, (CH), and hydroxymethylidyne (COH). Furthermore, and critical to the production of liquid fuels, are reactions that form C–C bonds, such as migratory insertion. Many related stoichiometric reactions have been simulated on discrete metal clusters, but homogeneous Fischer–Tropsch catalysts are of no commercial importance. Addition of isotopically labelled alcohol to the feed stream results in incorporation of alcohols into product. This observation establishes the facility of C–O bond scission. Using 14C-labelled ethylene and propene over cobalt catalysts results in incorporation of these olefins into the growing chain. Chain growth reaction thus appears to involve both ‘olefin insertion’ as well as ‘CO-insertion’. 8CO+17H2⟶C8H18+8H2O The extra high quantity of Hydrogen atoms are produced by the VIPTM subsystem, as a part of the patented TCG technology but patented separately as well.
Feedstocks: gasification
Fischer–Tropsch plants associated with biomass or coal or related solid feedstocks (sources of carbon) must first convert the solid fuel into gases. These gases include CO, H2, and alkanes. This conversion is called gasification. Synthesis gas (“syngas”) is obtained from biomass/coal gasification is a mixture of hydrogen and carbon monoxide. The H2:CO ratio is adjusted using the water-gas shift reaction. Coal-based FT plants produce varying amounts of CO2, depending upon the energy source of the gasification process. However, most coal-based plants rely on the feed coal to supply all the energy requirements of the process.
Feedstocks: GTL
Carbon monoxide for FT catalysis is derived from hydrocarbons. In gas to liquids (GTL) technology, the hydrocarbons are low molecular weight materials that often would be discarded or flared. Stranded gas provides relatively cheap gas. For GTL to be commercially viable, gas must remain relatively cheaper than oil. Several reactions are required to obtain the gaseous reactants required for FT catalysis. First, reactant gases entering a reactor must be desulfurized. Otherwise, sulfur-containing impurities deactivate (“poison”) the catalysts required for FT reactions. Several reactions are employed to adjust the H2:CO ratio. Most important is the water-gas shift reaction, which provides a source of hydrogen at the expense of carbon monoxide:
For FT plants that use methane as the feedstock, another important reaction is dry reforming, which converts the methane into CO and H2: CH4+CO2⟶2CO+2H2
Process conditions
Generally, the Fischer–Tropsch process is operated in the temperature range of 150–300 °C (302–572 °F). Higher temperatures lead to faster reactions and higher conversion rates but also tend to favor methane production. For this reason, the temperature is usually maintained at the low to middle part of the range. Increasing the pressure leads to higher conversion rates and also favors the formation of long-chained alkanes, both of which are desirable. Typical pressures range from one to several tens of atmospheres. Even higher pressures would be favorable, but the benefits may not justify the additional costs of high-pressure equipment, and higher pressures can lead to catalyst deactivation via coke formation. A variety of synthesis-gas compositions can be used. For cobalt-based catalysts the optimal H2:CO ratio is around 1.8–2.1. Iron-based catalysts can tolerate lower ratios, due to the intrinsic water-gas shift reaction activity of the iron catalyst. This reactivity can be important for synthesis gas derived from coal or biomass, which tend to have relatively low H2:CO ratios (< 1).